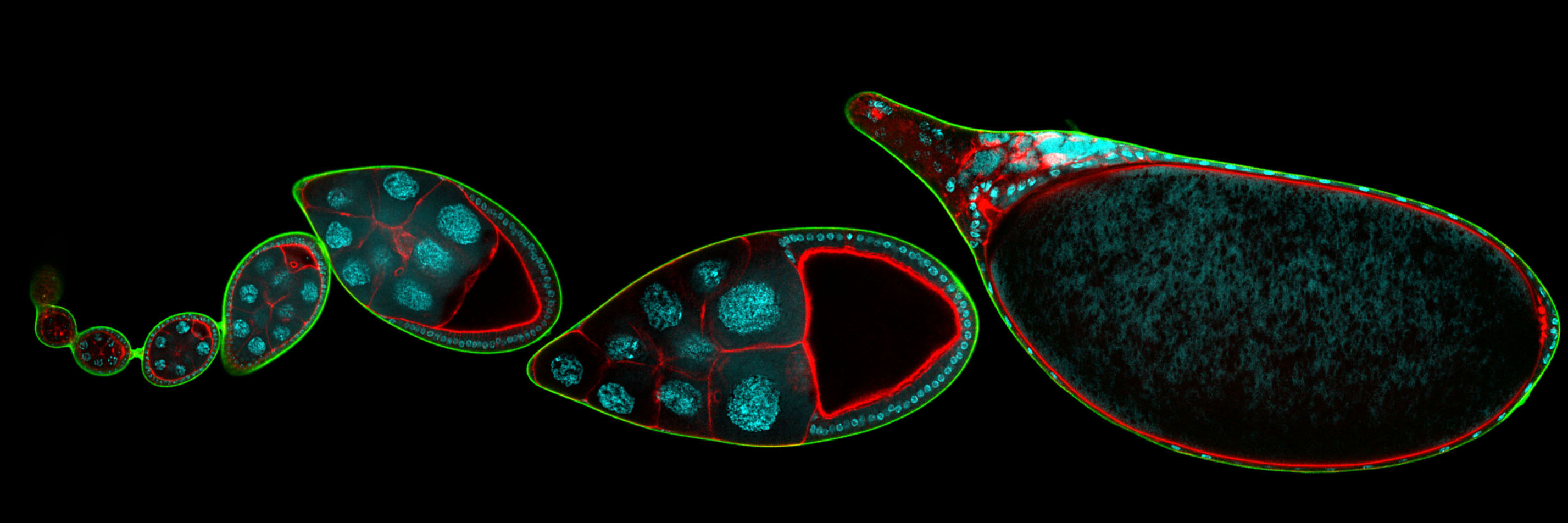
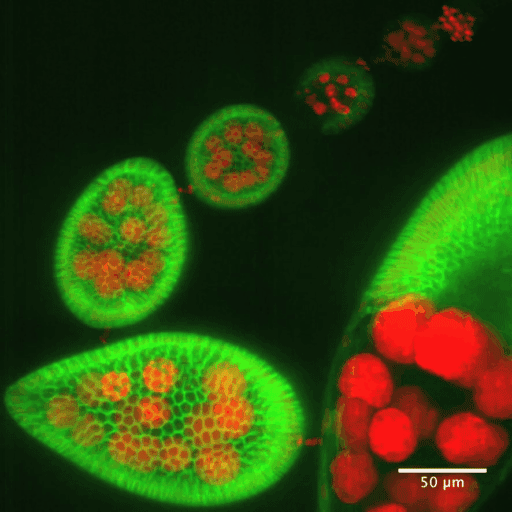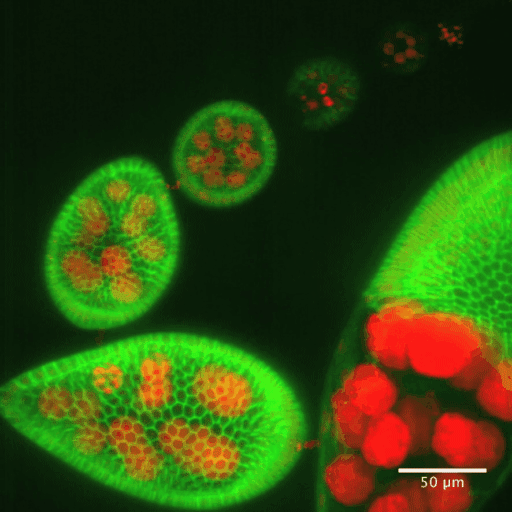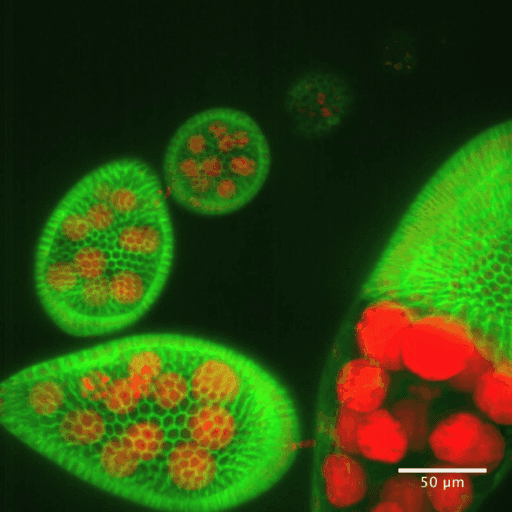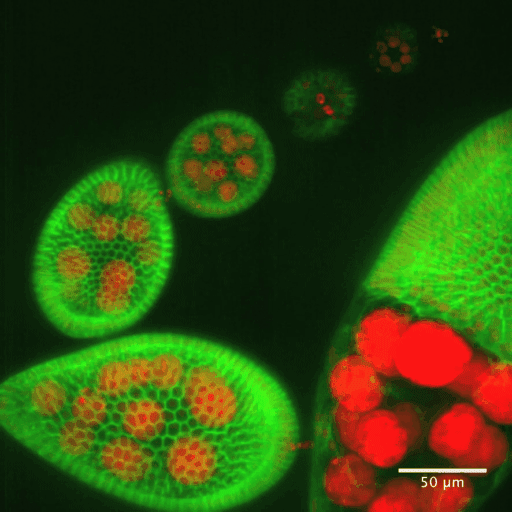
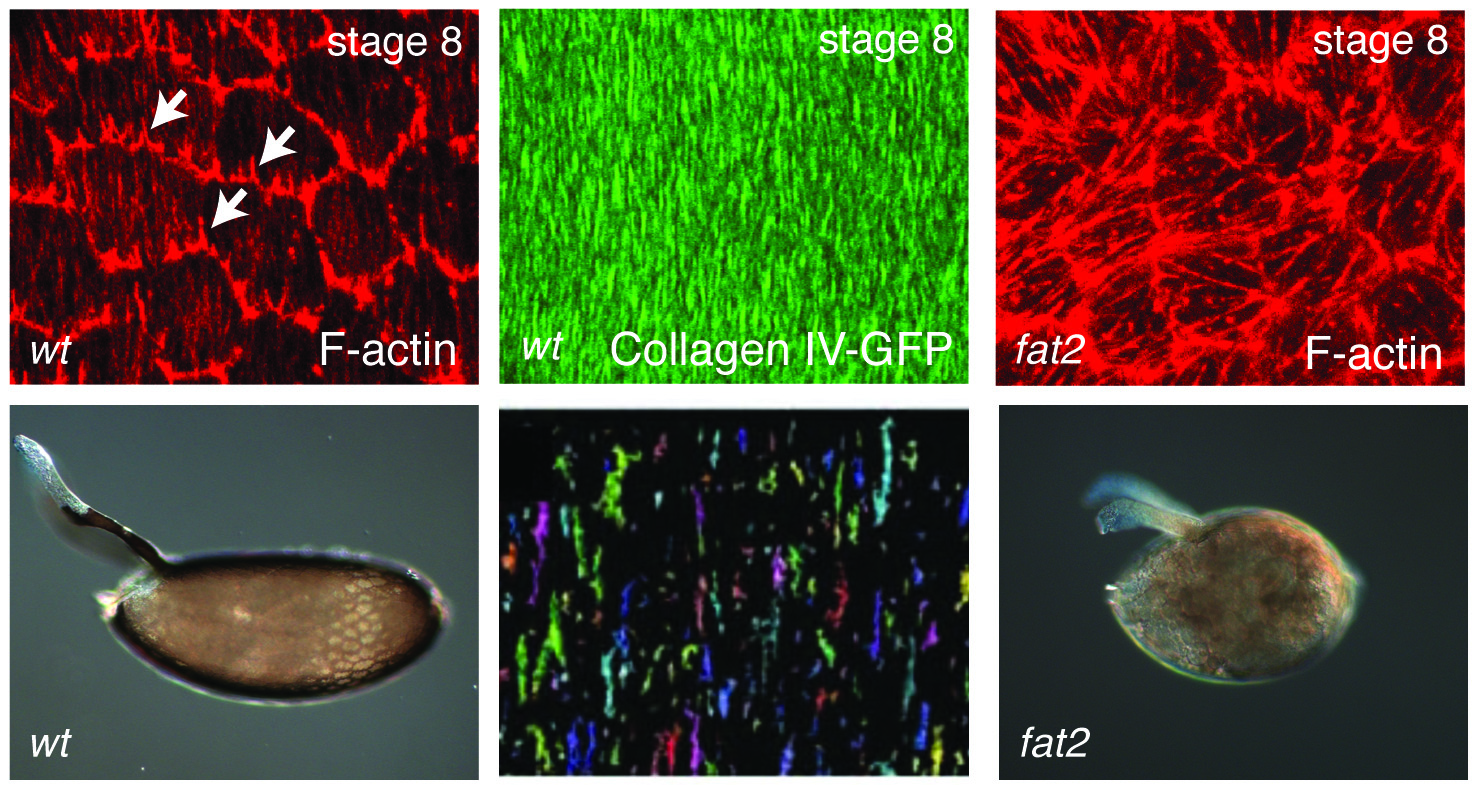
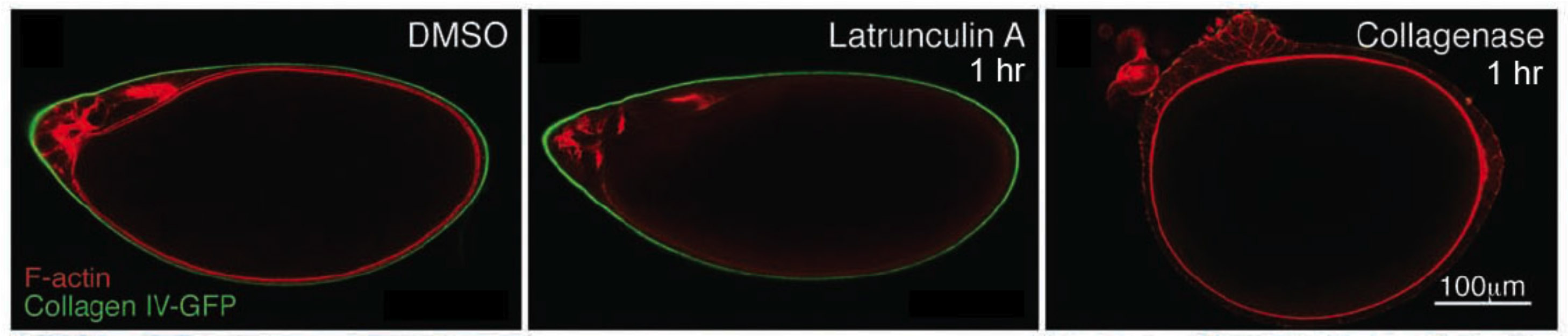
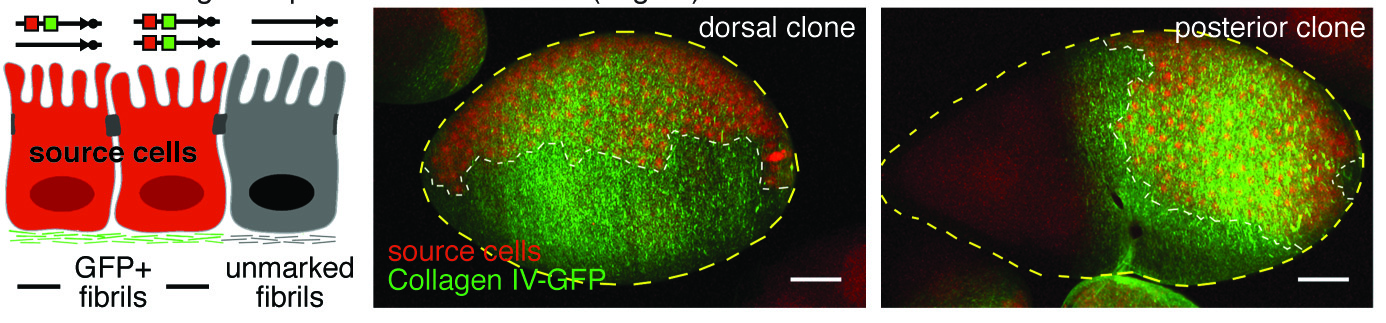
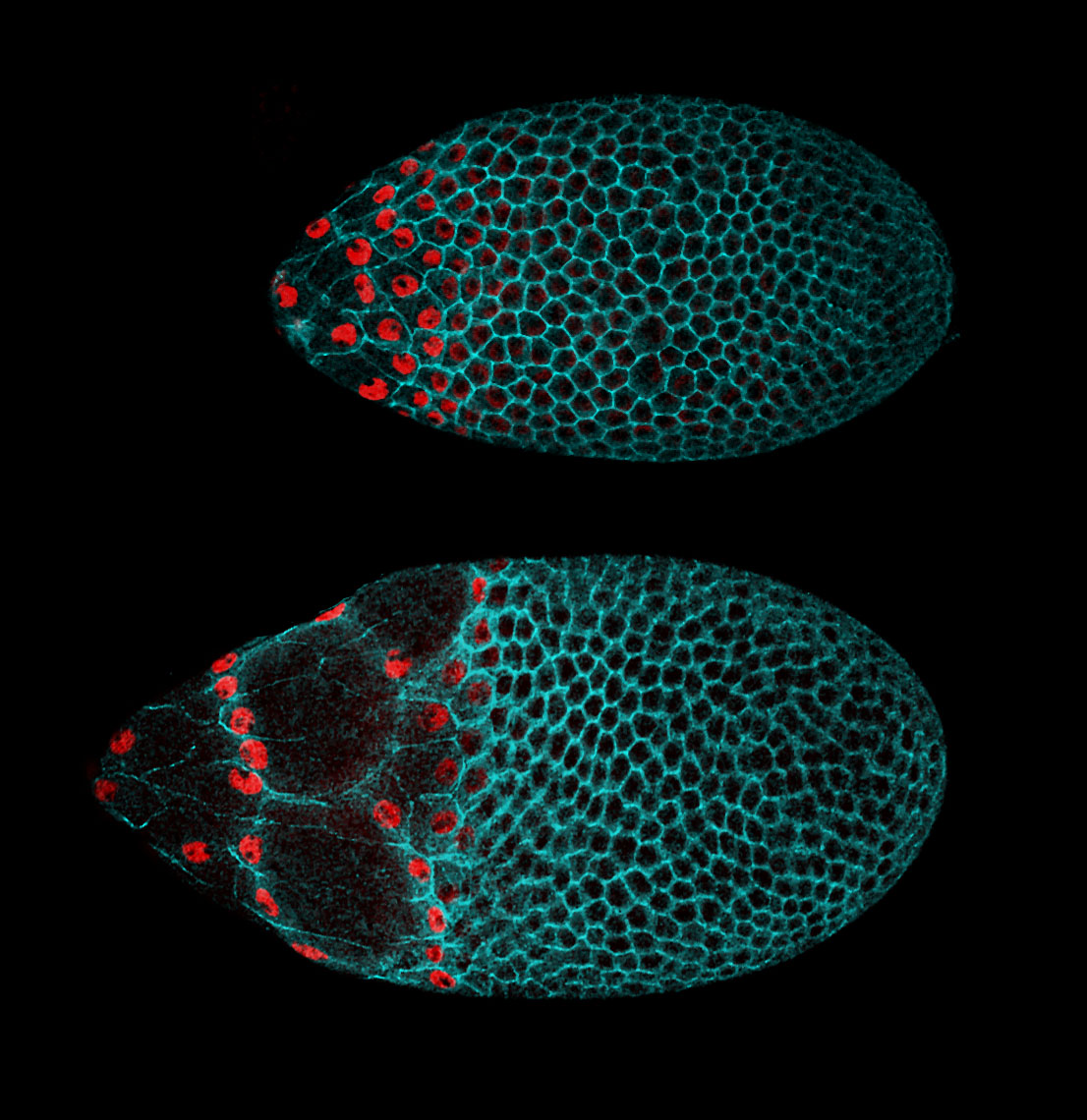
In addition to static aspects of epithelial architecture, we are interested in how epithelial cells and sheets organize themselves to drive the morphogenetic outcomes that shape organs and organisms. The follicle cell epithelium of the adult Drosophila ovary is a rich system for such studies. Follicle cells undergo numerous conserved morphogenetic behaviors, including transitions between squamous, cuboidal, and columnar morphologies, cellular rearrangements and migrations; these changes during development determine the final three-dimensional form of the mature egg. We are using cell biological, imaging, and biomechanical approaches in conjunction with forward genetic screening to understand the morphogenetic processes that shape this simple organ as a general model for understanding the processes and forces that govern epithelial morphogenesis.
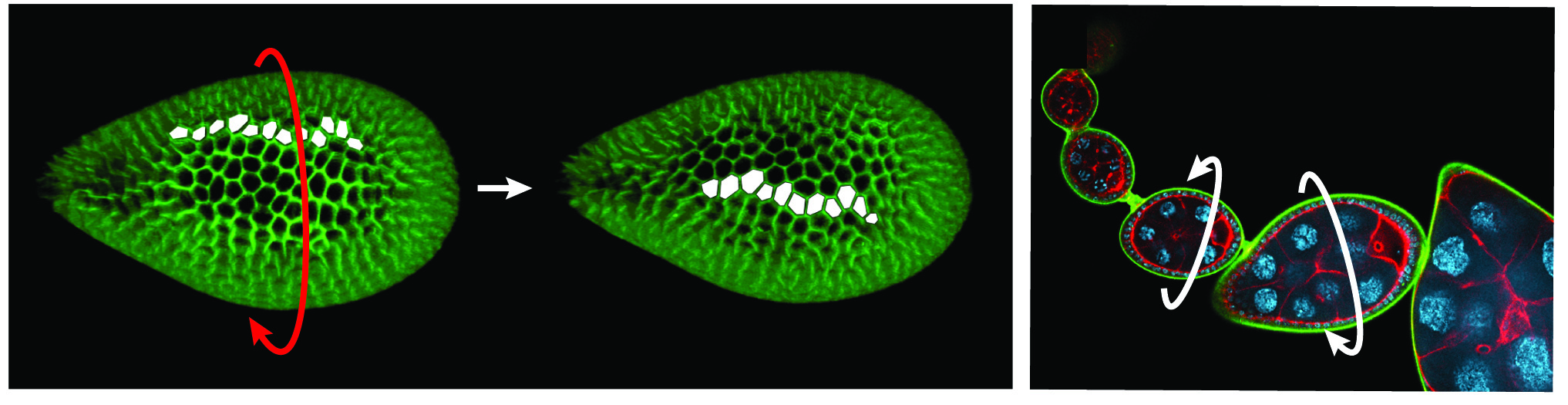
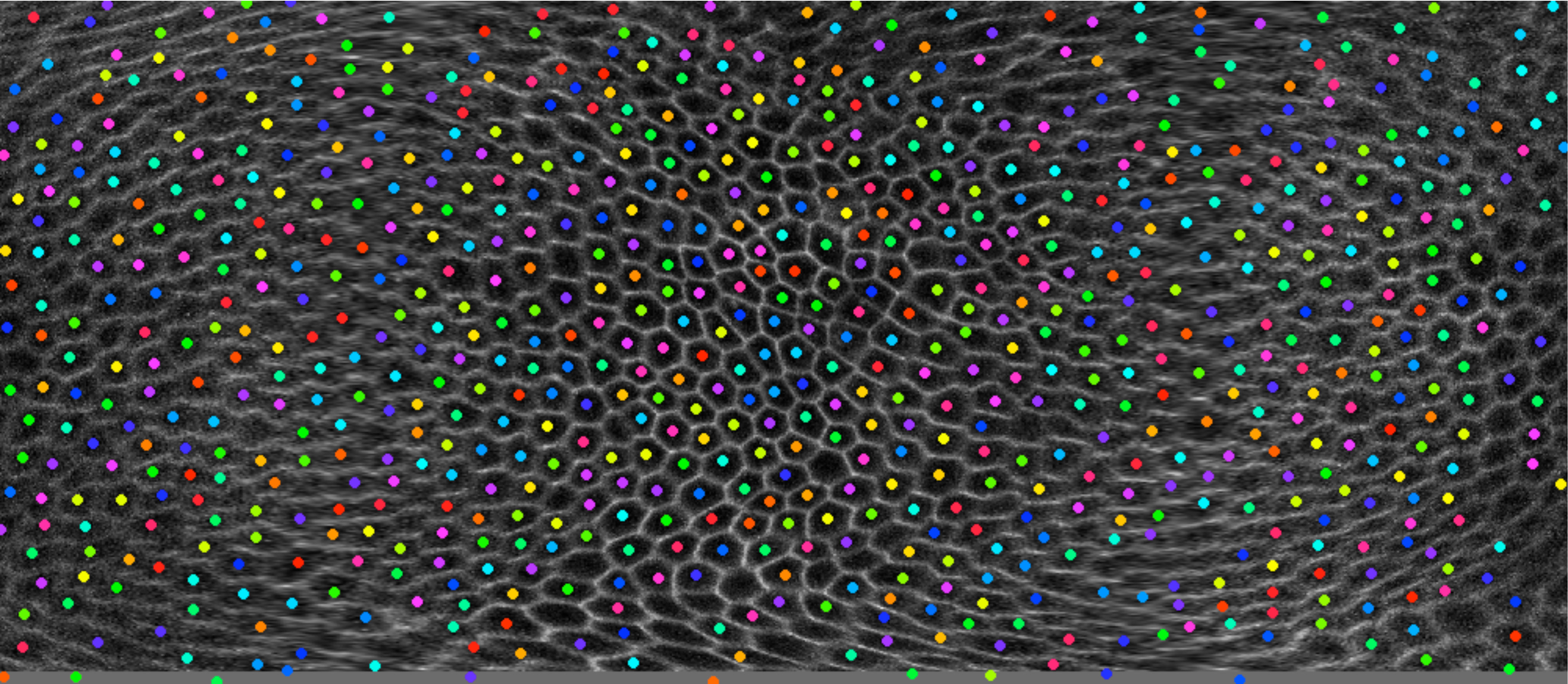
One current focus is the mechanisms underlying tissue elongation in the follicle. This elongation is an elemental morphogenetic change that requires activities in both the epithelium and the surrounding extracellular matrix (ECM). These activities include a dramatic, planar-polarized collective cell migration that rotates the entire tissue. We are studying the origin and coordination of this migration, the molecular mechanisms that coordinate planar polarity in this ‘edgeless epithelium’, and how they creates anisotropic biomechanical forces that influence epithelial and ECM properties to shape the organ.