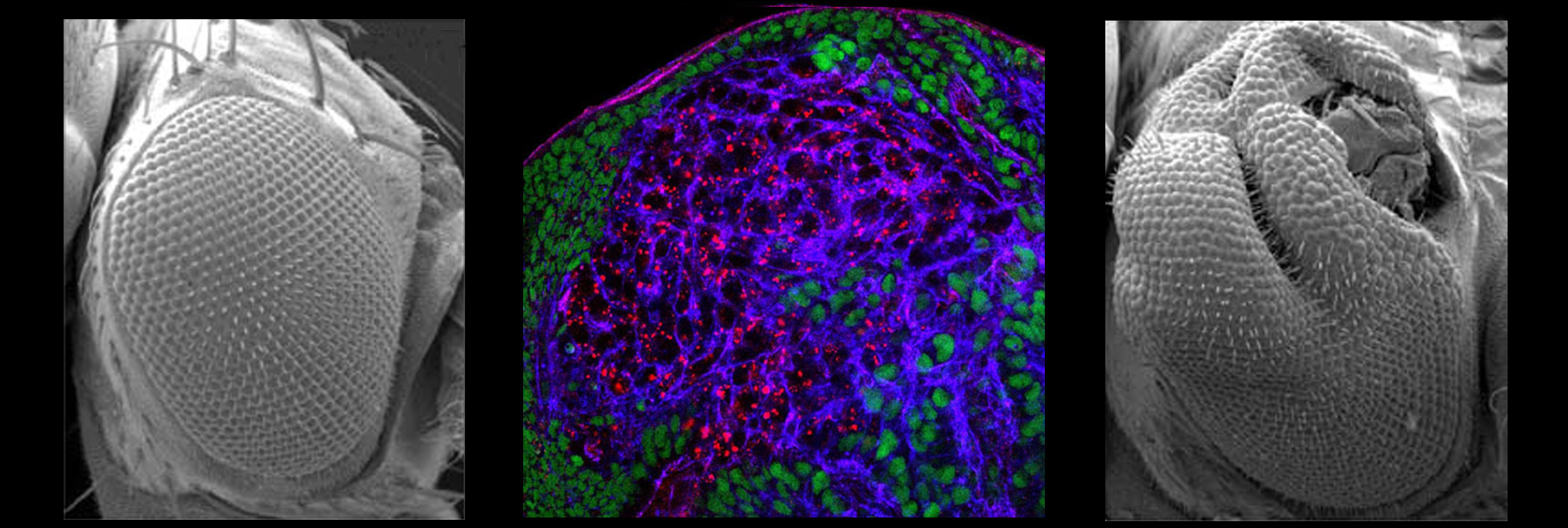
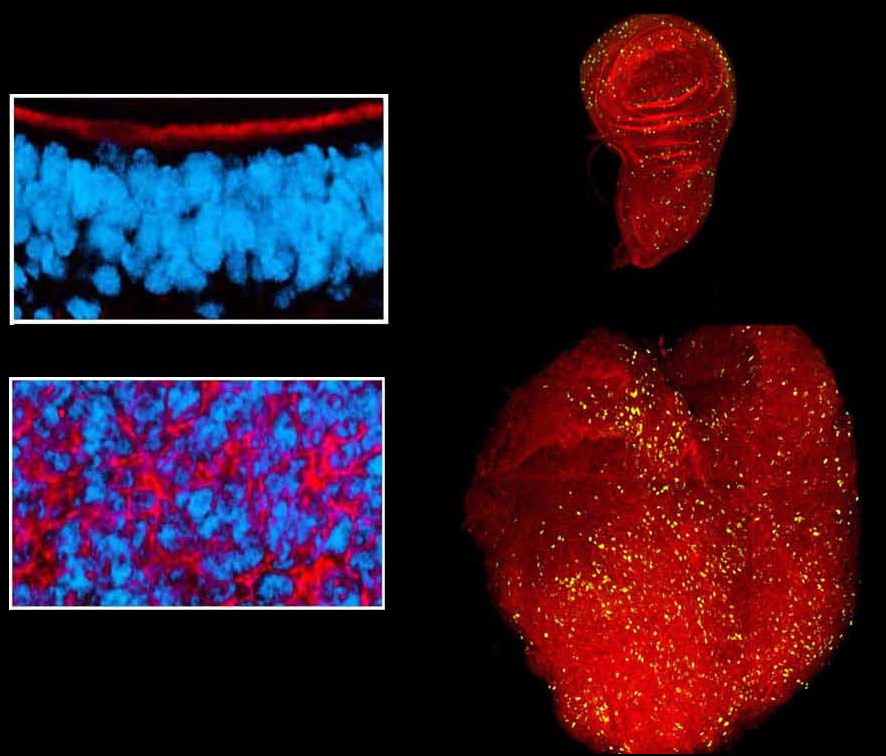
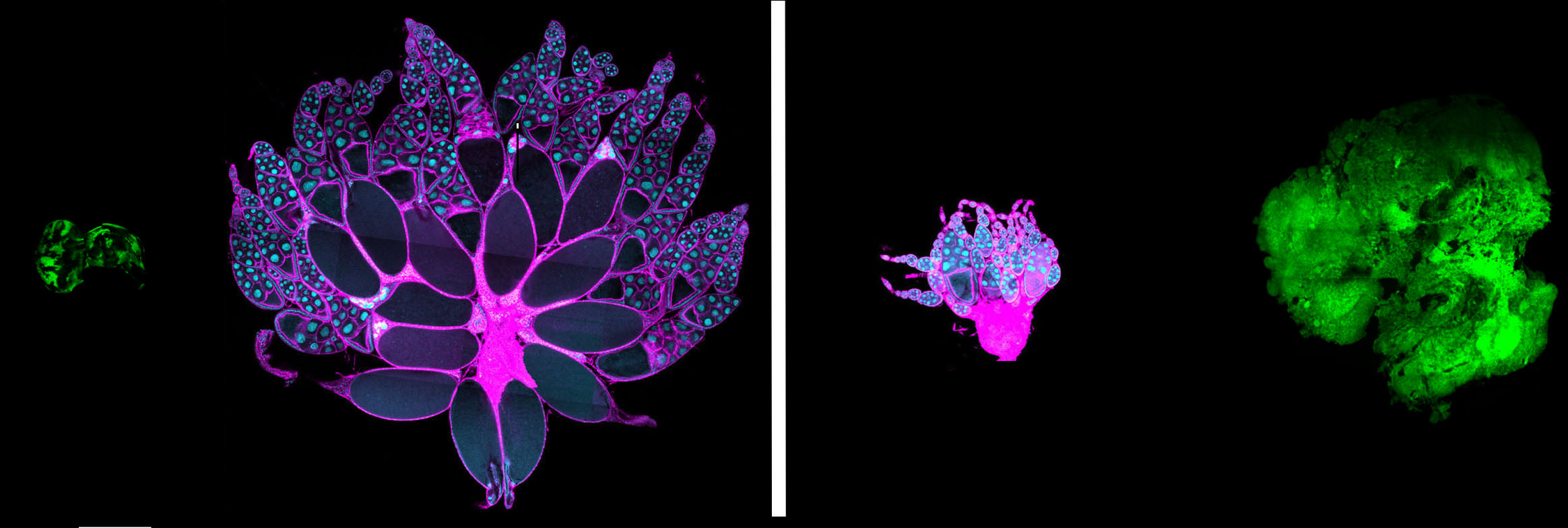
The final size of epithelial organs is tightly regulated during the development of many animals, and in insects in particular size control seems to be intrinsic to the organ itself. How epithelial cells communicate to each other to measure the size of the organ as a whole, and to make a communal decision to cease proliferation, is an outstanding mystery. We are exploring the unknown pathways by which organ size is controlled, and in particular how the unique features of epithelial architecture promote the restraint of excess growth.
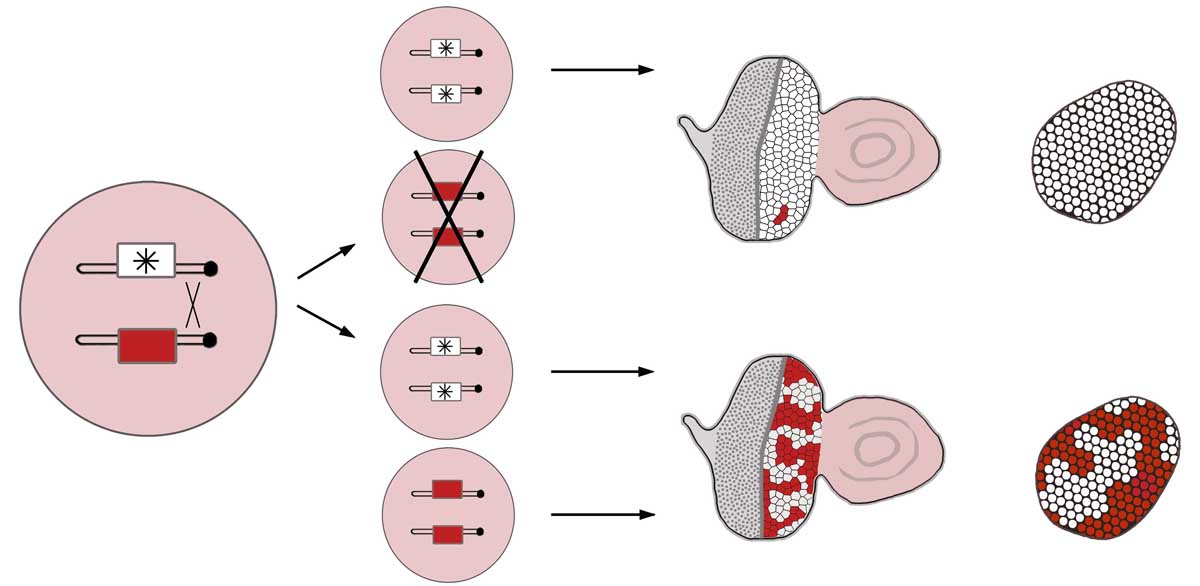
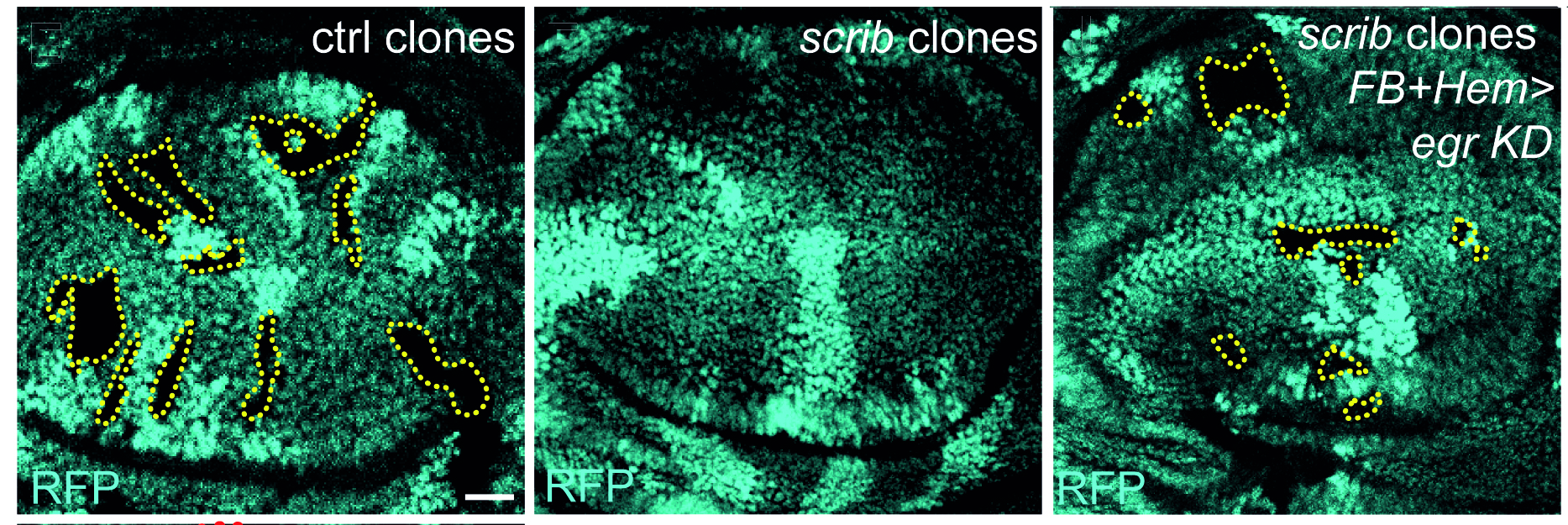
Cell polarity and proliferation appear coupled in a number of contexts, and disruption of epithelial polarity correlates with the progression of malignant tumors. The causal relationship or mechanism of such coupling has been obscure. We have advanced and framed the hypothesis that alterations of cell polarity can have a causative contribution to inappropriate cell division, and demonstrated that this is indeed true in the simple and experimentally amenable system of the Drosophila neoplastic tumor suppressor genes (TSGs). We are currently using this system to address a number of interesting questions, including: Why does exit from cell cycle and control of organ size require epithelial organization? What factors lie downstream of neoplastic tumor suppressors to affect growth, and what are the mechanisms underlying their inappropriate regulation? How do the pathways misregulated in tumorous growth relate to those that normally control wound healing and regeneration? Can the fly model shed light on mechanisms of human tumor formation and their lethality? These questions are being pursued through both hypothesis-based and discovery-driven approaches, including an efficient forward genetic screen that isolates new TSGs.
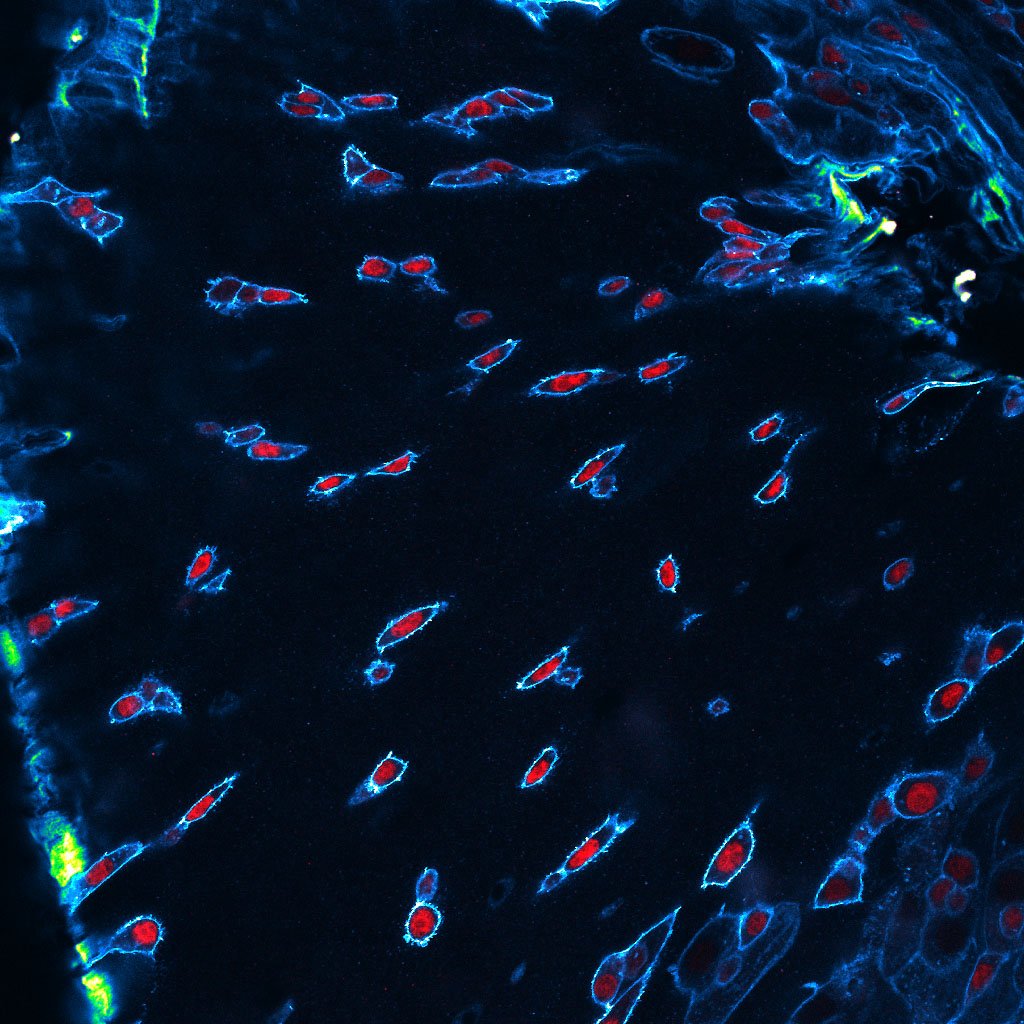
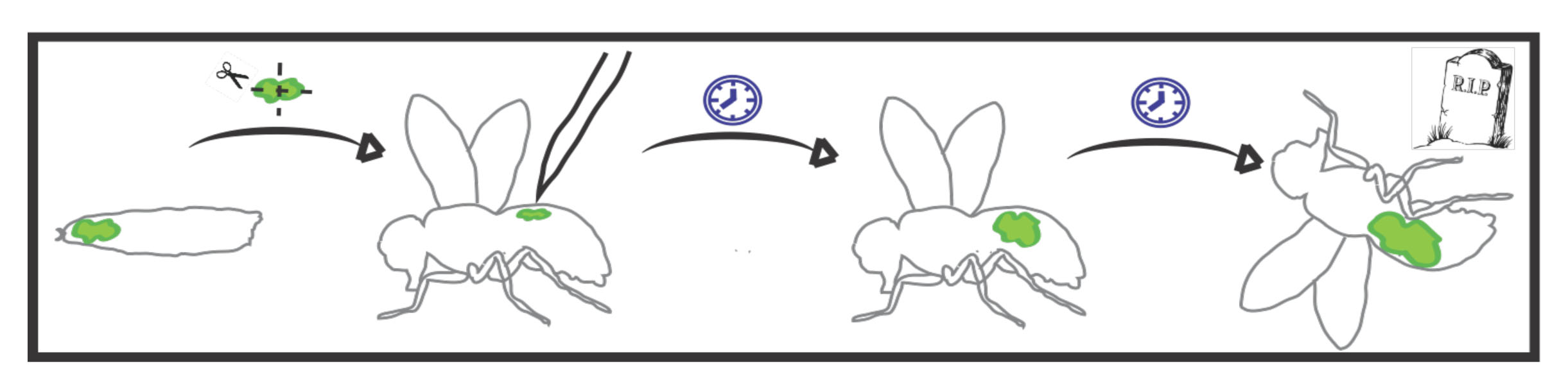
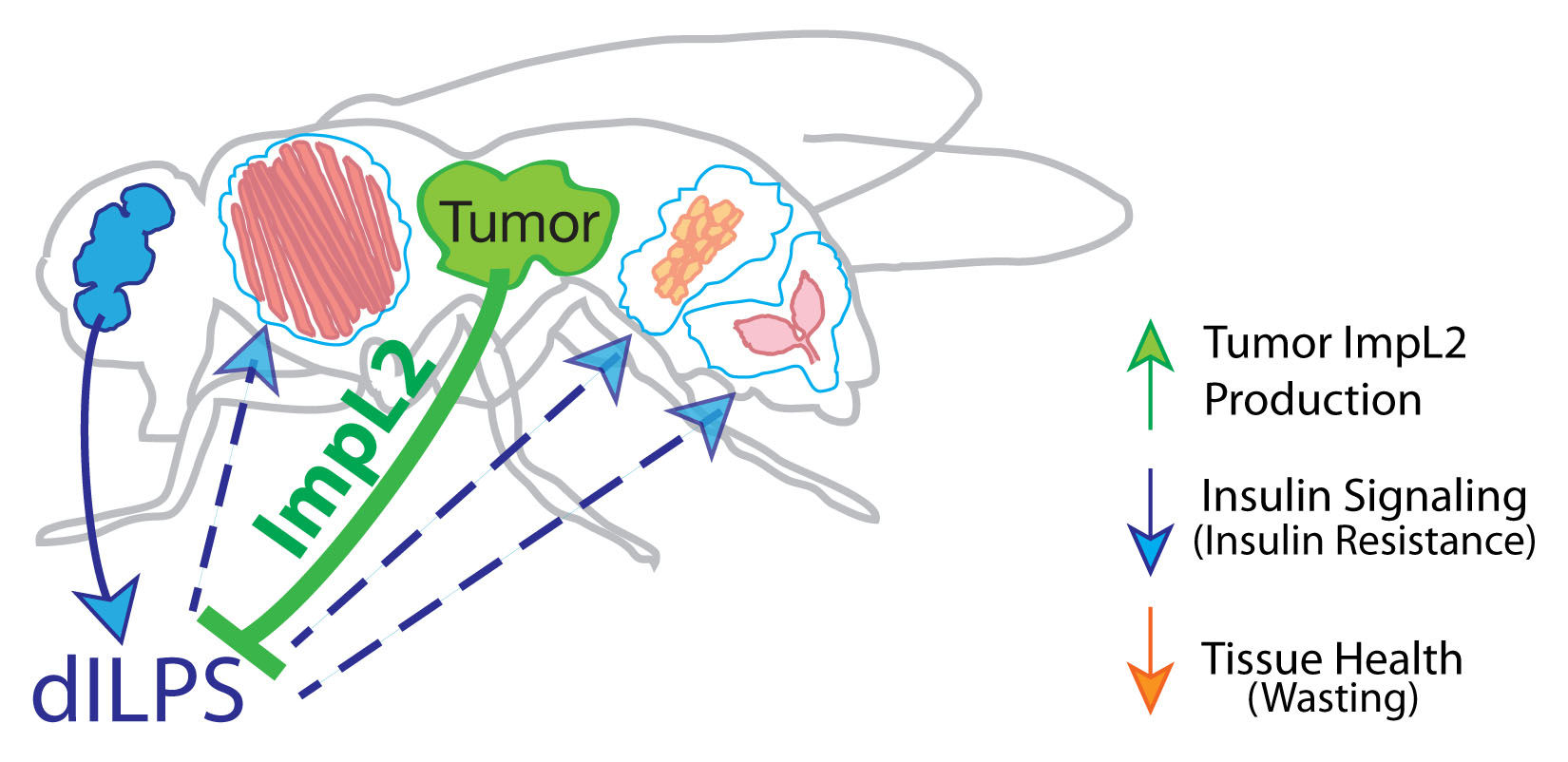
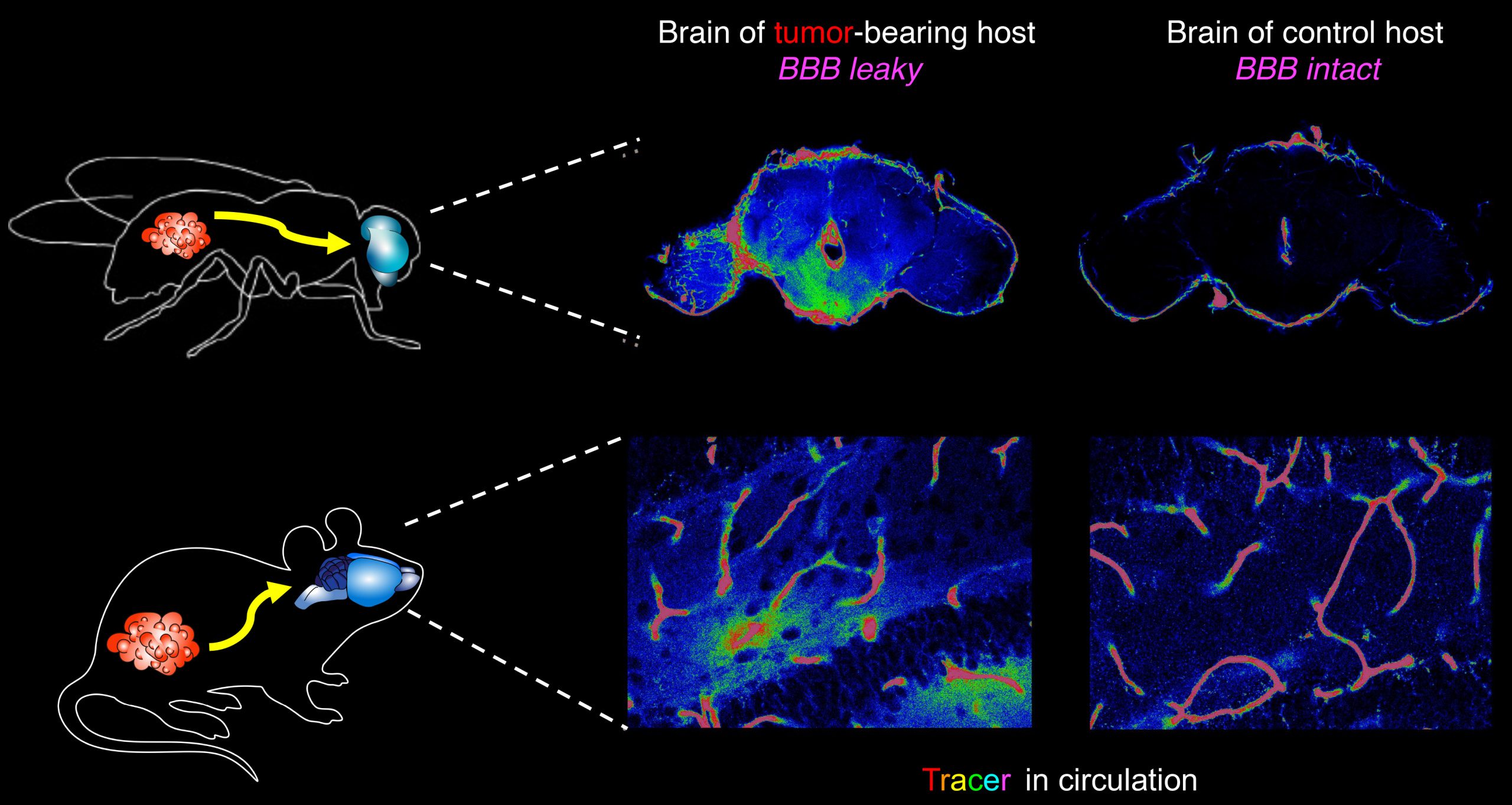
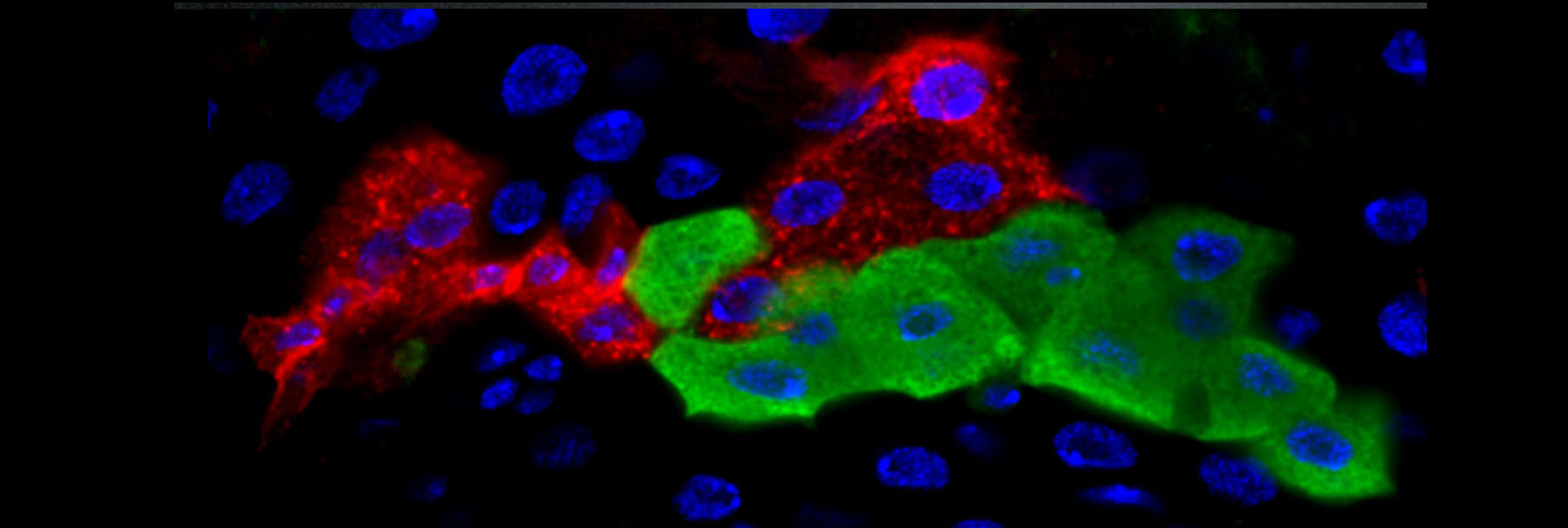
The lab’s newest direction leverages the many advantages of Drosophila to study tumor-host interactions, particularly systemic ‘paraneoplasias’ through which tumors impact host organs at a distance through pathological inter-organ communication. We have shown that flies exhibit cancer cachexia, and identified a molecular mediator that is upregulated in some cachectic human tumors. We have also used the fly to discover a completely novel paraneoplasia involving inflammatory opening of the blood-brain barrier, and collaborated to demonstrate that this is conserved in a mouse cancer model where it also regulates host mortality. Ongoing work is defining additional new tumor-host interactions and exploring how they contribute to the rapidly accelerated lethality seen in tumor-bearing flies.