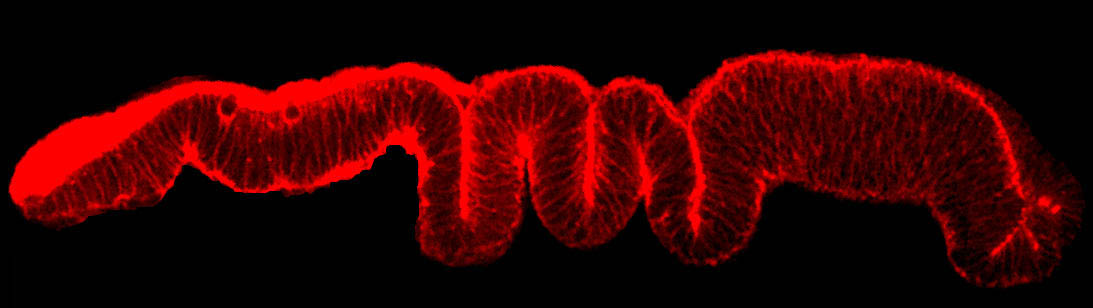
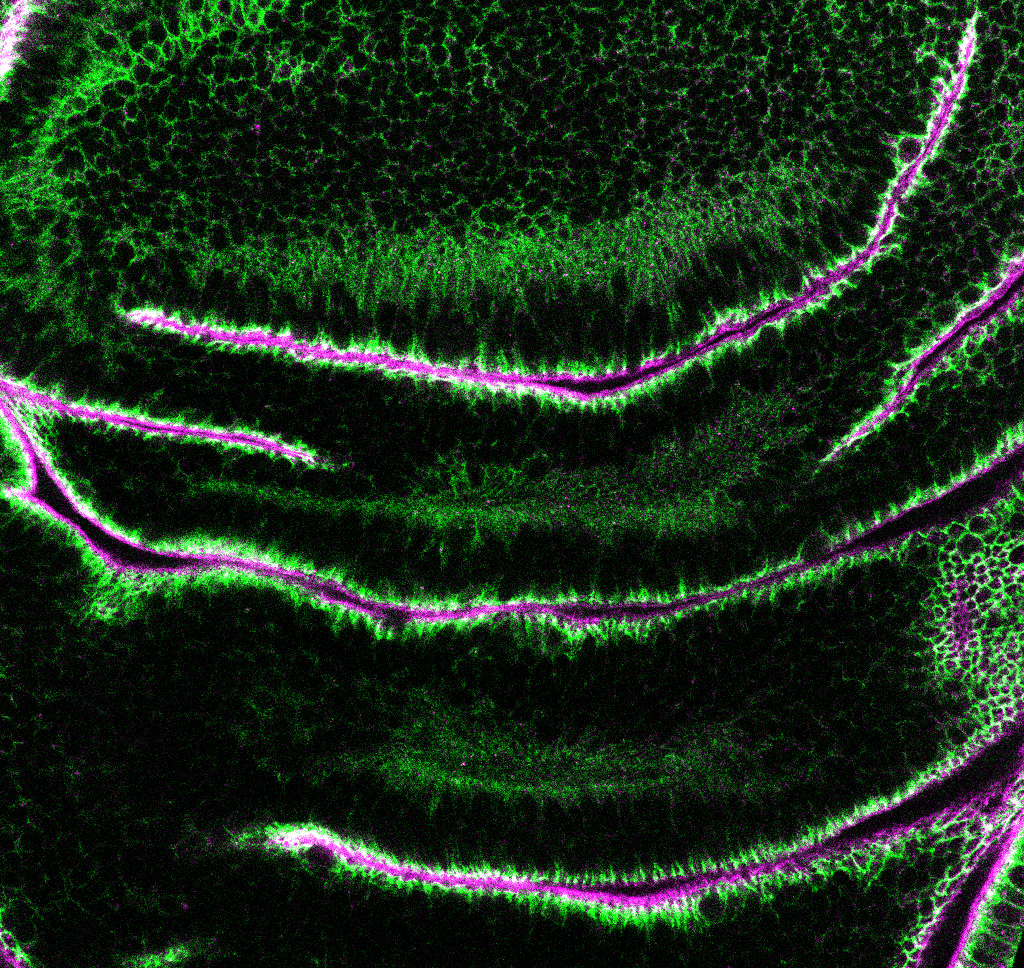
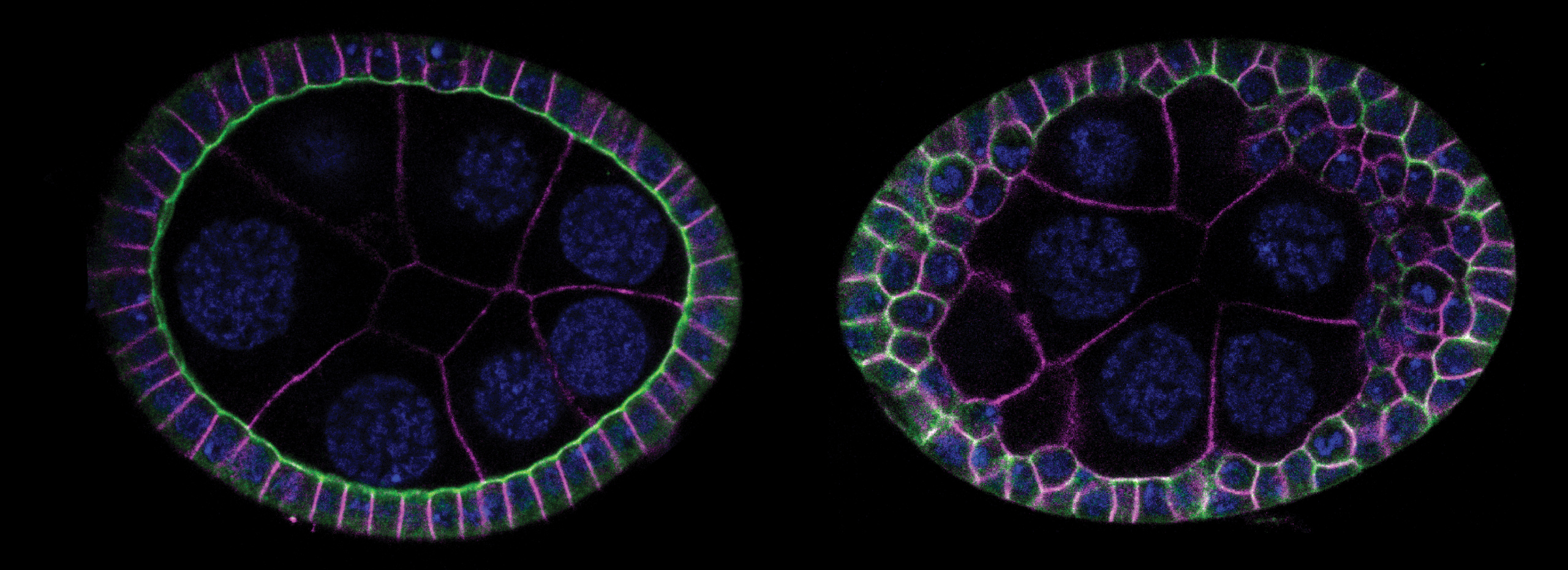
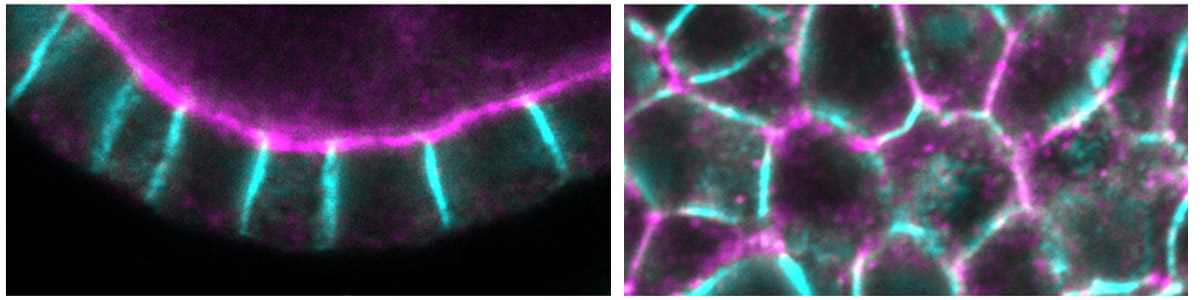
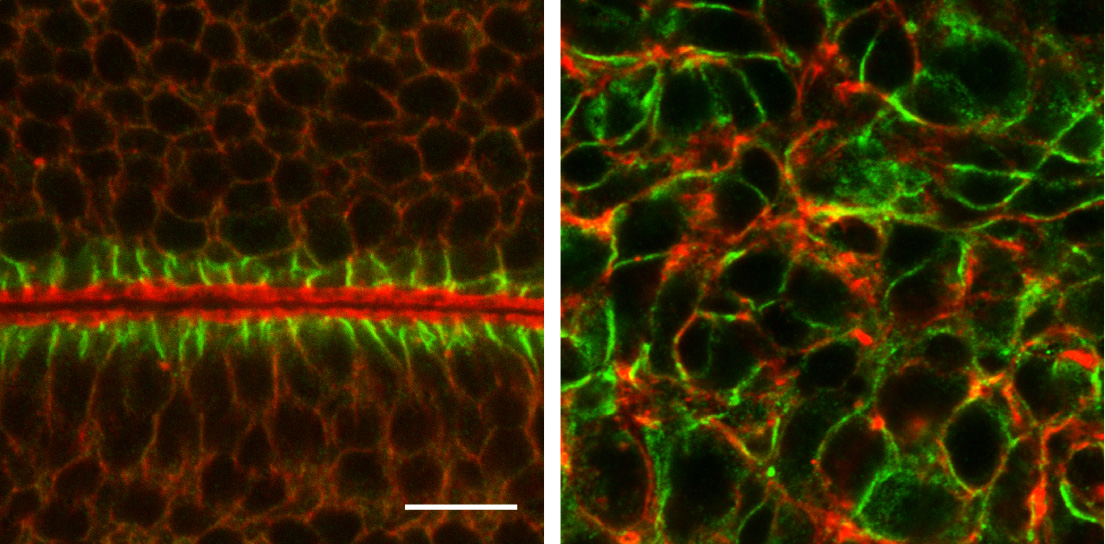
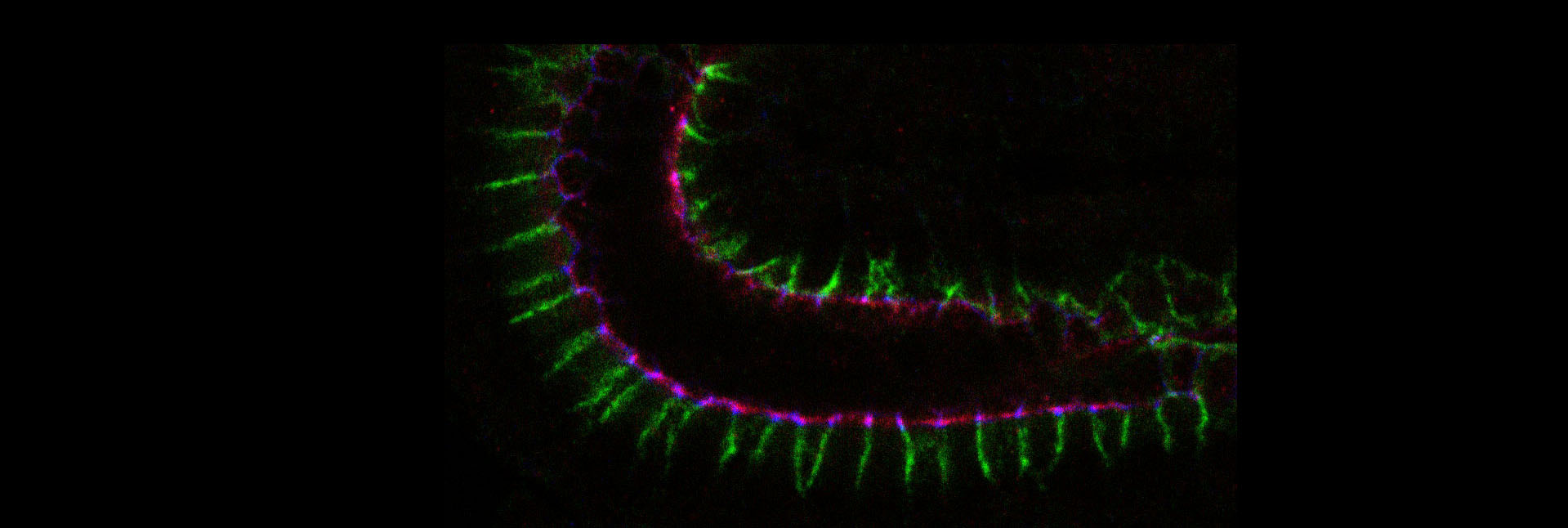
Epithelial cells constitute the most widespread and evolutionarily ancient mode of animal tissue organization. The functions of epithelia rely on their highly polarized architecture, in which specific proteins are restricted to apical, junctional, and basolateral surfaces. We are working to understand the mechanisms that give rise to and maintain this polarized distribution of membrane proteins, which epitomize subcellular specialization.
One line of investigation seeks to understand the cellular mechanism by which established polarity regulators control this polarized protein distribution. These regulators include three evolutionarily conserved groups of scaffolding proteins: the Par (-3/Par-6/aPKC) and Crumbs (/Stardust) complexes, which are found at the adherens junctions and apical surface respectively, and the Scribble (Discs-large/Lethal Giant Larvae) module that act at the basolateral surface. While increasingly well-characterized genetically and biochemically, their basic cellular polarizing activities remain mysterious.
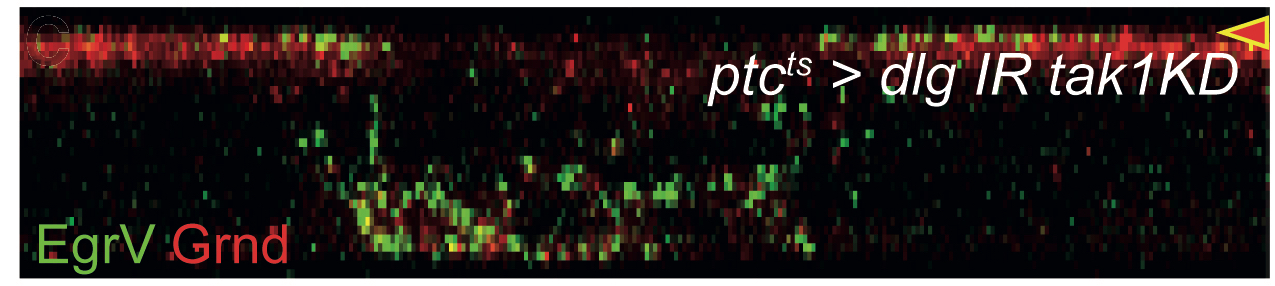
As novel insights can come from unbiased genetic screens, we have designed several to isolate new regulators of epithelial polarity. These screens identify genes that directly interface with the conserved polarity regulators, revealing mechanistic links with basic membrane trafficking, RNA localization, and protein degradation machineries. Using these and other genes as entry points, we are studying the cellular and molecular mechanisms underlying their various polarizing activities in order to expand existing paradigms of epithelial polarity and obtain an integrated picture of how the cell achieves the proper apicobasal distribution of proteins.